Galvanic Corrosion is created when metals of different potentials are attached to each other and submerged in water or any solution that carries electrical current. Since different metals corrode at different rates, a current flow will be created from the faster corroding metal (anode) to the slower corroding metal (cathode). A reliable anode must be capable of continuously supplying electrical current throughout its life. HARBOR anodes are made from high purity base metals before being alloyed with controlled amounts of additives to enhance electrochemical properties and to offset the small amount of impurities that detract from current output. Improper or unalloyed anodes start off with a high current output which tapers off over time, thus providing insufficient current for long term protection.
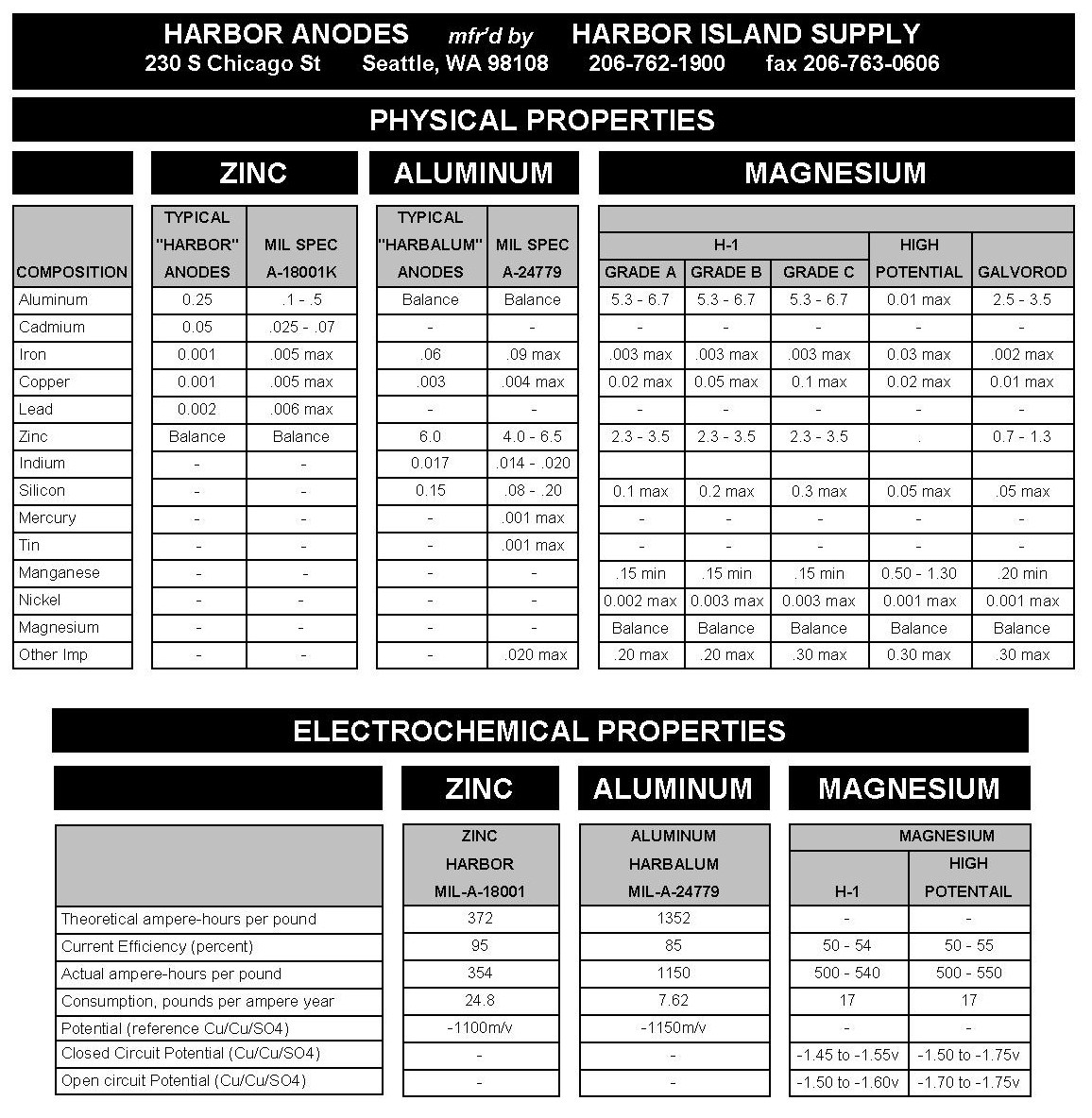
Differences between ZINC – ALUMINUM – MAGNESIUM Anodes
Zinc Anodes work well in salt and the upper saline levels of brackish waters. In lower levels of brackish and fresh waters, zinc doesn’t have the current output to fully protect, however, partial protection occurs.
Aluminum Anodes work well in salt and the upper saline levels of brackish waters. In lower levels of brackish and fresh waters, aluminum anodes can film over by not sloughing its consumed metal which causes passivation and the possibility of a total loss of galvanic protection.
Magnesium Anodes work well in salt, brackish and fresh waters. Fresh water use is more prevalent due to the possible “over-protection” that may occur in the less resistant environments of salt and upper saline level of brackish waters.
Summary: There are several factors that play into which type of anode to use on any given structure or vessel. These include water salinity, water temperature, water speed, metals to be protected, required anode life span, etc. For vessel protection, typical speed and how often the vessel is used can make a difference in choosing the proper protection. Changing habits in a vessel’s use can also change the type of anode required for protection. Since electrical currents change every time a vessel travels into different environments (waters), there is no absolute value to the corrosion rates of metals so each vessel (or structure) must be looked at individually for the proper protection.
ZINC Reference Cell “Interpretation Readings”
Protected Steel: CuCu/So4 @ -.85v Protected Steel: Zinc Reference Cell @ -.25v
METAL
Magnesium
Zinc
Aluminum
Cadmium
Steel
Cast Iron
Aluminum Bronze
Tin
Copper
Tin/Lead Solder
Brass
Manganese Bronze
Silicon Bronze
Copper/Nickel
Lead
Silver
Titanium
Platinum
Graphite
NATURAL READINGS
-.350 to -.550
+.050 to -.150
+.050 to -.150
+.050 to -.150
+.350 to +.550
+.350 to +.550
+.400 to +.600
+.400 to +.600
+.400 to +.600
+.450 to +.650
+.450 to +.650
+.450 to +.650
+.500 to +.750
+.500 to +.750
+.650 to +.850
+.650 to +.850
+.700 to +.900
+.950 to +1.150
+1.05 to +1.250
PROTECTED READINGS
-.550 to -.750
-.150 to -.350
-.150 to -.350
-.150 to -.350
+.150 to +.350
+.150 to +.350
+.200 to +.400
+.200 to +.400
+.200 to +.400
+.250 to +.450
+.250 to +.450
+.250 to +.450
+.300 to +.550
+.300 to +.550
+.450 to +.650
+.450 to +.650
+.500 to +.700
+.750 to +.950
+.850 to +1.050